Signal transduction
In biology, signal transduction is a mechanism that converts a mechanical/chemical stimulus to a cell into a specific cellular response.[1] Signal transduction starts with a signal to a receptor, and ends with a change in cell function.
Transmembrane receptors span the cell membrane, with part of the receptor outside and part inside the cell. The chemical signal binds to the outer portion of the receptor, changing its shape and conveying another signal inside the cell. Some chemical messengers, such as testosterone, can pass through the cell membrane, and bind directly to receptors in the cytoplasm or nucleus.
Sometimes there is a cascade of signals within the cell. With each step of the cascade, the signal can be amplified, so a small signal can result in a large response.[1] Eventually, the signal creates a change in the cell, either in the expression of the DNA in the nucleus or in the activity of enzymes in the cytoplasm.
These processes can take milliseconds (for ion flux), minutes (for protein- and lipid-mediated kinase cascades), hours, or days (for gene expression).
Contents |
Signaling molecules
Most signal transduction involves the binding of extracellular signaling molecules (and ligands) to cell-surface receptors. Such receptors typically face outward from the plasma membrane while triggering events inside the cell. Intracellular signaling cascades can also be triggered through cell-substratum interactions, as in the case of integrins, which bind ligands found within the extracellular matrix. Steroids represent another example of extracellular signaling molecules that may cross the plasma membrane due to their lipophilic or hydrophobic nature.[2] Many, but not all, steroid hormones have receptors within the cytoplasm, and usually act by stimulating the binding of their receptors to the promoter region of steroid-responsive genes.[3] Within multicellular organisms, numerous small molecules and polypeptides serve to coordinate a cell's individual biological activity within the context of the organism as a whole. These molecules have been functionally classified as:
- hormones (e.g., melatonin),[4]
- growth factors (e.g. epidermal growth factor),[5]
- extra-cellular matrix components (e.g., fibronectin),[6]
- cytokines (e.g., interferon-gamma),[7]
- chemokines (e.g., RANTES),[8]
- neurotransmitters (e.g., acetylcholine),[9] and
- neurotrophins (e.g., nerve growth factor).[10]
- active oxygen species and other electronically-activated compounds (see redox signaling).
Most of these classifications do not take into account the molecular nature of each class member. For example, as a class, neurotransmitters consist of neuropeptides such as endorphins[11] and small molecules such as serotonin[12] and dopamine.[13] Hormones, another generic class of molecules capable of initiating signal transduction, include insulin (a polypeptide),[14] testosterone (a steroid),[15] and epinephrine (an amino acid derivative, in essence a small organic molecule).[16]
The classification of one molecule into one class or another is not exact. For example, epinephrine and norepinephrine, secreted by the central nervous system, act as neurotransmitters. However, epinephrine when secreted by the adrenal medulla acts as a hormone.
Environmental stimuli
In bacteria and other single-cell organisms, the variety of a signal transduction processes of which the cell is capable influences how many ways it can react and respond to its environment. In multicellular organisms, numerous signal transduction processes are required for coordinating the behavior of individual cells to support the function of the organism as a whole. The complexity of an organism's signal transduction processes tends to increase with the complexity of the organism itself. Sensing of both the external and internal environments at the cellular level relies on signal transduction. Many disease processes, such as diabetes, heart disease, autoimmunity, and cancer arise from defects in signal transduction pathways, further highlighting the critical importance of signal transduction to biology, as well as medicine.
Various environmental stimuli, in addition to many of the regular signal transduction stimuli listed above, initiate signal transmission processes in complex organisms. Environmental stimuli may also be molecular in nature (as above) or more physical, such as light striking cells in the retina of the eye,[17] odorants binding to odorant receptors in the nasal epithelium,[18] and bitter and sweet tastes stimulating taste receptors in the taste buds.[19]
Certain microbial molecules, e.g., viral nucleotides, bacterial lipopolysaccharides, and protein antigens, are able to elicit an immune system response against invading pathogens, mediated by signal transduction processes. An immune response may occur independent of signal transduction stimulation by other molecules, as is the case for signal transduction by way of the Toll-like receptor or with help from stimulatory molecules located at the cell surface of other cells, as is the case for T-cell receptor signaling.
Unicellular organisms may also respond to environmental stimuli through the activation of signal transduction pathways. For example, slime molds secrete cyclic-AMP upon starvation, which stimulates individual cells in the immediate environment to aggregate.[20] Yeast use mating factors to determine the mating types of other yeast and to participate in sexual reproduction.[21]
Cellular responses
Activation of genes,[22] alterations in metabolism,[23] the continued proliferation and death of the cell,[24] and the stimulation or suppression of locomotion,[25] are some of the cellular responses to extracellular stimulation that require signal transduction. Gene activation leads to further cellular effects, since the protein products of many of the responding genes include enzymes and transcription factors themselves. Transcription factors produced as a result of a signal transduction cascade can, in turn, activate yet more genes. Therefore an initial stimulus can trigger the expression of an entire cohort of genes, and this, in turn, can lead to the activation of any number of complex physiological events. These events include the increased uptake of glucose from the blood stream stimulated by insulin[23] and the migration of neutrophils to sites of infection stimulated by bacterial products. The set of genes and the order in which they are activated in response to stimuli are often referred to as a genetic program.[26]
Neurotransmitters are ligands that are capable of binding to ion channel proteins, resulting in their opening to allow the rapid flow of a particular ion across the plasma membrane.[9] This results in an altering of the cell's membrane potential and is important for processes such as the neural conduction of electrochemical impulses. Ligands can be freely soluble,[5] or can be found on the surface of other cells or within the extracellular matrix.[6] Such cell surface or extracellular matrix ligands signal between cells when they come in contact with each other, such as when a phagocytic cell presents antigens to lymphocytes, or upon adhesion to the extracellular matrix, as when integrins at the cell surface of fibroblasts engage fibronectin.[27]
Most mammalian cells require stimulation to control not only cell division but also survival. In the absence of growth factor stimulation, programmed cell death ensues in most cells. Such requirements for extra-cellular stimulation are necessary for controlling cell behavior in the context of both unicellular and multi-cellular organisms. Signal transduction pathways are perceived to be so central to biological processes that it is not surprising that a large number of diseases have been attributed to their disregulation.
Discussed below are how signal transduction via various classes of receptor may lead to the above cellular responses.
Types of receptor
Receptors can be roughly divided into two major classes:
- Intracellular receptors and
- Cell-surface receptors.
Ligand-gated ion channel receptors are a class of receptor that may occur both at the cell-surface or intracellularly.
Solely intracellular receptors include those for steroid hormones, thyroid hormone, retinoic acid, and derivatives of vitamin D3. In contrast to ligands that bind to cell surface receptors to initiate signal transduction, these ligands must cross the cell membrane. See the intracellular receptors section below for more details.
Major categories of intracellular receptors include G-protein linked receptors and Tyrosine Kinase receptors.
Cell-surface receptors
Cell-surface receptors are integral transmembrane proteins and recognize the vast majority of extracellular signaling molecules. Transmembrane receptors span the plasma membrane of the cell, with one part of the receptor on the outside of the cell (the extracellular domain), and the other on the inside of the cell (the intracellular domain). Signal transduction occurs as a result of stimulatory molecule or the binding of a ligand to its extracellular domain; the ligand itself does not pass through the plasma membrane prior to receptor-binding.
Binding of a ligand to a cell-surface receptor stimulates a series of events inside the cell, with different types of receptor stimulation of different intracellular responses. Receptors typically respond to only the binding of a specific ligand. Upon binding, the ligand initiates the transmission of a signal across the plasma membrane by inducing a change in the shape or conformation of the intracellular part of the receptor (see this link [2] for a molecular model for receptor activation). Often, such changes in conformation either result in the activation of an enzymatic activity contained within the receptor or expose a binding site for other signaling proteins within the cell. Once these proteins bind to the receptor, they themselves may become active and propagate the signal into the cytoplasm.
In eukaryotic cells, most intracellular proteins activated by a ligand/receptor interaction possess an enzymatic activity. These enzymes include tyrosine kinase, heterotrimeric G proteins, small GTPases, various serine/threoine protein kinases, phosphatases, lipid kinases, and hydrolases. Some receptor-stimulated enzymes create specific second messengers including cyclic nucleotides, such as cyclic AMP (cAMP) and cyclic GMP (cGMP), Phosphatidylinositol derivatives, such as Phosphatidylinositol-triphosphate (PIP3), Diacylglycerol (DAG) and Inositol-triphosphate (IP3), IP3, controlling the release of intracellular calcium stores into the cytoplasm (see second messengers section later in this article). Other activated proteins interact with adapter proteins. Adapter proteins facilitate interactions between other signaling proteins, and coordinate the formation of signaling complexes necessary to produce an appropriate cellular response to a particular stimulus. Enzymes and adapter proteins are both responsive to various second messenger molecules.
Many of the enzymes activated as part of the signal transduction mechanism and also many adapter proteins have been found to possess specialized protein domains that bind to specific secondary messenger molecules. For example, calcium ions bind specifically to the EF hand domains of calmodulin, allowing this molecule to bind and activate Calmodulin-dependent kinase. PIP3, PIP2 and other phosphoinositides may bind to the Pleckstrin homology domains of proteins such as the kinase protein AKT again with activation activity.
There are many different classes of transmembrane receptor that recognize different extracellular signaling molecules. Specific example receptors discussed in this article are:
- G-protein coupled receptors, e.g., Chemokine receptors
- Receptor tyrosine kinases, e.g., Growth factor receptors,
- Integrins
- Toll-like receptors
Further examples are given in the transmembrane receptor article.
G-protein-coupled receptors
.png)
G-protein-coupled receptors (GPCRs) are a family of integral membrane proteins that possess seven membrane-spanning domains, and are linked to a guanine nucleotide-binding protein (or heterotrimeric G protein). Many receptors make up this family, including adrenergic receptors, neurotransmitter receptors, olfactory receptors, opioid receptors, chemokine receptors, and rhodopsin.
Signal transduction by a GPCR begins with an inactive G protein coupled to the receptor. An inactive G protein exists as a heterotrimer, a molecule composed of three different protein subunits: Gα, Gβ, and Gγ. Once the GPCR recognizes a ligand, the shape (conformation) of the receptor changes to mechanically activate the G protein, and causes one subunit (Gα) to bind a molecule of GTP (causing activation) and dissociate from the other two G-protein subunits (Gβ and Gγ). The dissociation exposes sites on the G-protein subunits that interact with other molecules.[28] The activated G protein subunits detach from the receptor and initiate signaling from many downstream effector proteins. These include phosphodiesterases and adenylyl cyclases, phospholipases, and ion channels that permit the release of second messenger molecules such as cyclic-AMP (cAMP), cyclic-GMP (cGMP), inositol triphosphate (IP3), diacylglycerol (DAG), and calcium (Ca2+) ions.[29] For example, a rhodopsin molecule in the plasma membrane of a retina cell in the eye that was activated by a photon can activate up to 2000 effector molecules (in this case, transducin) per second.
The total strength of signal amplification by a GPCR is determined by:
- The lifetime of the ligand-receptor-complex. If the ligand-receptor-complex is stable, it takes longer for the ligand to dissociate from its receptor, thus the receptor will remain active for longer and will activate more effector proteins.
- The amount and lifetime of the receptor-effector protein-complex. The more effector protein is available to be activated by the receptor, and the faster the activated effector protein can dissociate from the receptor, the more effector protein will be activated in the same amount of time.
- Deactivation of the activated receptor. A receptor that is engaged in a hormone-receptor-complex can be deactivated, either by covalent modification (for example, phosphorylation) or by internalization (see ubiquitin).
- Deactivation of effectors through intrinsic enzymatic activity. Either small or large G-proteins possess intrinsic GTPase activity, which controls the duration of the triggered signal. This activity may be increased through the action of other proteins such as GTPase-activating proteins (GAPS).
The idea that G-protein-coupled receptors, to be specific, chemokine receptors, participate in cancer development is suggested by a study wherein a point mutation was inserted into the gene encoding the chemokine receptor CXCR2. Cells transfected with the CXCR2 mutant underwent a malignant transformation.[30] The result of the point mutation was the expression of CXCR2 in an active conformation, despite the absence of chemokine-binding (the CXCR2 mutant is said to be "constitutively active").
Receptor tyrosine kinases
Receptor tyrosine kinases (RTKs) are transmembrane proteins with an intracellular kinase domain and an extracellular domain that binds ligand. There are many RTK proteins that are classified into subfamilies depending on their structural properties and ligand specificity. These include many growth factor receptors such as insulin receptor and the insulin-like growth factor receptors, and many others receptors.[31] To conduct their biochemical signals, RTKs need to form dimers in the plasma membrane.[32] The dimer is stabilized by ligand binding by the receptor. Interaction between the two cytoplasmic domains of the dimer is thought to stimulate autophosphorylation of tyrosines within the cytoplasmic tyrosine kinase domains of the RTKs causing their conformational changes. The kinase domain of the receptors is subsequently activated, initiating signaling cascades of phosphorylation of downstream cytoplasmic molecules. These signals are essential to various cellular processes, such as control of cell growth, differentiation, metabolism, and migration.[31]
As is the case with G-Protein-coupled receptors, proteins that bind GTP play a major role in transmission of signal from the activated RTK into the cell. In this case, the G proteins are members of the Ras, Rho, and Raf families, referred to collectively as small G proteins. These proteins act as molecular switches that are usually tethered to membranes by isoprenyl groups linked to their carboxyl ends. Thus, upon activation, they are responsible for the recruitment of proteins to specific membrane subdomains where they participate in signaling. Activated RTKs, in turn, activate small G proteins, which in turn activate Guanine Nucleotide Exchange Factors, such as SOS1. Once activated, these exchange factors can activate many more small G-proteins, thus amplifying the receptors initial signal.
As with the mutation of G-protein coupled receptors, the mutation of certain RTK genes can result in the expression of receptors that exist in a constitutively-activate state. Such mutated RTK genes may act as oncogenes, genes that contribute to the initiation or progression of cancer.[33]
Integrins
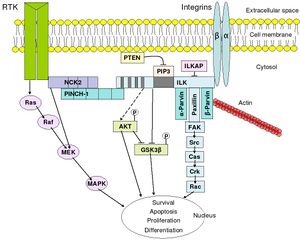
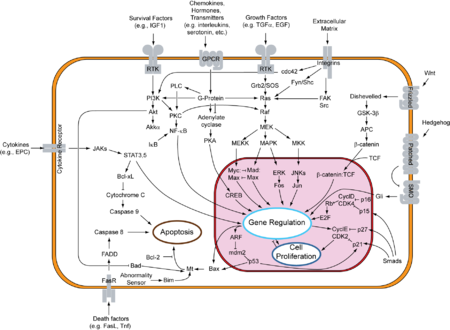
Integrins are produced by a wide variety of cell types, and play a role in the attachment of a cell to the extracellular matrix (ECM) and to other cells, and in the signal transduction of signals received from extracellular matrix components such as fibronectin, collagen, and laminin. Ligand-binding to the extracellular domain of integrins induces a conformational change within the protein and a clustering of the protein at the cell surface to initiate signal transduction. Integrins lack kinase activity, and integrin-mediated signal transduction is achieved through a variety of intracellular protein kinases and adaptor molecules such as integrin-linked kinase (ILK), focal-adhesion kinase (FAK), talin, paxillin, parvins, p130Cas, Src-family kinases, and GTPases of the Rho family, the main protein coordinating signal transduction being ILK.[34] As shown in the overview to the right, cooperative integrin and receptor tyrosine kinase signaling determine cellular survival, apoptosis, proliferation, and differentiation.
Important differences exist between integrin-signaling in circulating blood cells and that in non-circulating blood cells such as epithelial cells. Integrins at the cell-surface of circulating cells are inactive under normal physiological conditions. For example, cell-surface integrins on circulating leukocytes are maintained in an inactive state to avoid epithelial cell attachment. Only in response to appropriate stimuli are leukocyte integrins converted into an active form, such as those received at the site of an inflammatory response. In a similar manner, it is important that integrins at the cell surface of circulating platelets are kept in an inactive state under normal conditions to avoid thrombosis. Epithelial cells, in contrast, have active integrins at their cell surface under normal conditions, which help maintain their stable adhesion to underlying stromal cells, which provide appropriate signals to maintain their survival and differentiation.[35]
Toll-like receptors
When activated, Toll-like receptors (TLRs) recruit adapter molecules within the cytoplasm of cells in order to propagate a signal. Four adapter molecules are known to be involved in signaling. These proteins are known as MyD88, Tirap (also called Mal), Trif, and Tram.[36][37][38] The adapters activate other molecules within the cell, including certain protein kinases (IRAK1, IRAK4, TBK1, and IKKi) that amplify the signal, and ultimately lead to the induction or suppression of genes that orchestrate the inflammatory response. In all, thousands of genes are activated by TLR signaling, and, together, the TLRs constitute one of the most powerful and important gateways for gene modulation.
Ligand-gated ion channel receptors
A ligand-activated ion channel will recognize its ligand, and then undergo a structural change that opens a gap (channel) in the plasma membrane through which ions can pass. These ions will then relay the signal. An example for this mechanism is found in the receiving cell, or post-synaptic cell of a neural synapse.
By contrast, other ion channels open in response to a change in cell potential, that is, the difference of the electrical charge across the membrane. In neurons, this mechanism underlies the action potentials that travel along nerves. The influx of ions that occurs in response to ligand-gated ion channels often induce action potentials by depolarizing the membrane of the post-synaptic cells, which results in the wave-like opening of voltage-gated ion channels. In addition, calcium ions are also commonly allowed into the cell during ligand-induced ion channel opening. This calcium can act as a classical second messenger, setting in motion signal transduction cascades and altering the cellular physiology of the responding cell. This may result in strengthening of the synapse between the pre- and post-synaptic cells by remodeling the dendritic spines involved in the synapse.
Intracellular receptors
Intracellular receptors include nuclear receptors and cytoplasmic receptors, and are soluble proteins localized within the nucleoplasm or the cytoplasm, respectively. The typical ligands for nuclear receptors are lipophilic hormones, with steroid hormones (for example, testosterone, progesterone, and cortisol) and derivatives of vitamin A and D among them. To reach its receptor and initiate signal transduction, the hormone must pass through the plasma membrane, usually by passive diffusion. The nuclear receptors are ligand-activated transcription activators; on binding with the ligand (the hormone), the ligands will pass through the nuclear membrane into the nucleus and enable the transcription of a certain gene and, thus, the production of a protein.
The nuclear receptors that were activated by the hormones attach at the DNA at receptor-specific Hormone-Responsive Elements (HREs), DNA sequences that are located in the promoter region of the genes that are activated by the hormone-receptor complex. As this enables the transcription of the according gene, these hormones are also called inductors of gene expression. The activation of gene transcription is much slower than signals that directly affect existing proteins. As a consequence, the effects of hormones that use nucleic receptors are usually long-term. Although the signal transduction via these soluble receptors involves only a few proteins, the details of gene regulation are yet not well understood. The nucleic receptors all have a similar, modular structure:
- N-AAAABBBBCCCCDDDDEEEEFFFF-C
where CCCC is the DNA-binding domain that contains zinc fingers, and EEEE the ligand-binding domain. The latter is also responsible for dimerization of most nuclearic receptors prior to DNA binding. As a third function, it contains structural elements that are responsible for transactivation, used for communication with the translational apparatus. The zinc fingers in the DNA-binding domain stabilize DNA binding by holding contact to the phosphate backbone of the DNA. The DNA sequences that match the receptor are usually hexameric repeats, either normal, inverted, or everted. The sequences are quite similar, but their orientation and distance are the parameters by which the DNA-binding domains of the receptors can tell them apart.
Steroid receptors are a subclass of nuclear receptors, located primarily within the cytosol. In the absence of steroid hormone, the receptors cling together in a complex called an aporeceptor complex, which also contains chaperone proteins (also known as heatshock proteins or Hsps). The Hsps are necessary to activate the receptor by assisting the protein to fold in a way such that the signal sequence that enables its passage into the nucleus is accessible.
Steroid receptors can also have a repressive effect on gene expression, when their transactivation domain is hidden so it cannot activate transcription. Furthermore, steroid receptor activity can be enhanced by phosphorylation of serine residues at their N-terminal end, as a result of another signal transduction pathway, for example, a by a growth factor. This behaviour is called crosstalk.
RXR- and orphan-receptors These nuclear receptors can be activated by
- a classic endocrine-synthesized hormone that entered the cell by diffusion
- a hormone that was built within the cell (for example, retinol) from a precursor or prohormone, which can be brought to the cell through the bloodstream
- a hormone that was completely synthesized within the cell, for example, prostaglandin.
These receptors are located in the nucleus and are not accompanied by chaperone proteins. In the absence of hormone, they bind to their specific DNA sequence, repressing the gene. Upon activation by the hormone, they activate the transcription of the gene that they were repressing.
Certain intracellular receptors of the immune system are examples of cytoplasmic receptors. Recently-identified NOD like receptors (NLRs) reside in the cytoplasm of specific eukaryotic cells and interact with particular ligands, such as microbial molecules, using a leucine-rich repeat (LRR) motif that is similar to the ligand-binding motif of the extracellular receptors known as TLRs. Some of these molecules (e.g., NOD1 and NOD2) interact with an enzyme called RICK kinase (or RIP2 kinase) that activates NF-κB signaling, whereas others (e.g., NALP3) interact with inflammatory caspases (e.g., caspase 1) and initiate processing of particular cytokines (e.g., interleukin-1β).[39] Similar receptors exist inside plant cells and are called Plant R Proteins. Another type of cytoplasmic receptor also has a role in immune surveillance. These receptors are known as RNA Helicases and include RIG-I, MDA5, and LGP2.[40]
Second messengers
Intracellular signal transduction is largely carried out by second messenger molecules.
Calcium
Ca2+ concentration is usually maintained at a very low level in the cytosol by sequestration in the smooth endoplasmic reticulum and the mitochondria. Ca2+ release from the endoplasmic reticulum into the cytosol results in the binding of the released Ca2+ to signaling proteins that are then activated. There are two combined receptor/ion channel proteins that perform the task of controlled transport of Ca2+:
- The InsP3-receptor will transport Ca2+ upon interaction with inositol triphosphate (thus the name) on its cytosolic side. It consists of four identical subunits.
- The ryanodine receptor is named after the plant alkaloid ryanodine. It is similar to the InsP3 receptor and stimulated to transport Ca2+ into the cytosol by recognizing Ca2+ on its cytosolic side, thus establishing a feedback mechanism; a small amount of Ca2+ in the cytosol near the receptor will cause it to release even more Ca2+. It is especially important in neurons and muscle cells. In heart and pancreas cells, another second messenger (cyclic-ADP ribose) takes part in the receptor activation. The localized and time-limited activity of Ca2+ in the cytosol is also called a Ca2+ wave. Once released into the cytosol from intracellular stores or extracellular sources, Ca2+ acts as a signal molecule within the cell. This works by tightly limiting the time and space when Ca2+ is free (and thus active). Therefore, the concentration of free Ca2+ within the cell is usually very low; it is stored within organelles, usually the endoplasmic reticulum (sarcoplasmic reticulum in muscle cells), where it is bound to molecules like calreticulin.
Ca2+ is used in a multitude of processes, among them muscle contraction, release of neurotransmitter from nerve endings, vision in retina cells, proliferation, secretion, cytoskeleton management, cell migration, gene expression, and metabolism. The three main pathways that lead to Ca2+ activation are :
- G protein-regulated pathways
- Pathways regulated by receptor-tyrosine kinases
- Ligand- or current-regulated ion channels
There are two different ways by which Ca2+ can regulate proteins:
- A direct recognition of Ca2+ by the protein
- Binding of Ca2+ in the active site of an enzyme.
One of the best-studied interactions of Ca2+ with a protein is the regulation of calmodulin by Ca2+. Calmodulin itself can regulate other proteins, or be part of a larger protein (for example, phosphorylase kinase). The Ca2+/calmodulin complex plays an important role in proliferation, mitosis, and neural signal transduction.
Lipophilic
Lipophilic second messenger molecules are derived from lipids that normally reside in cellular membranes. Enzymes stimulated by activated receptors modify the lipids, converting them into second messengers.
Diacylglycerol is a lipophilic second messenger, required for the activation of protein kinase C. Ceramide, the eicosanoids, and lysophosphatidic acid are also lipophilic second messengers.
Nitric oxide
Nitric oxide (NO) can act as a second messenger. Nitric oxide gas is a free radical that diffuses through the plasma membrane and affects nearby cells. NO is made from arginine and oxygen by the enzyme NO synthase, with citrulline as a by-product. NO works mainly through activation of its target receptor, the enzyme soluble guanylate cyclase, which, when activated, produces the second messenger cyclic-guanosine monophosphate (cGMP). NO can also act through covalent modification of proteins or their metal co-factors. Some of these modifications are reversible and work through a redox mechanism. NO is toxic in high concentrations, and is thought to cause damage during stroke.
NO is involved in a number of functions, including relaxation of blood vessels; regulation of exocytosis of neurotransmitters; cellular immune response; modulation of the Hair Cycle; production and maintenance of penile erections; and activation of apoptosis by initiating signals that lead to H2AX phosphorylation.
Major pathway examples
.png)
- cAMP dependent pathway: In humans, cAMP works by activating protein kinase A (PKA, cAMP-dependent protein kinase) (see picture), and thus, further effects mainly depend on cAMP-dependent protein kinase, which vary based on the type of cell.
- MAPK/ERK pathway: A pathway that couples intracellular responses to the binding of growth factors to cell surface receptors. This pathway is very complex and includes many protein components.[42] The basic pathway shown in the figure (to the right) and described below includes the major components of the pathway. In many cell types, activation of this pathway promotes cell division.
- IP3/DAG pathway: PLC cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) yielding diacyl glycerol (DAG) and inositol 1,4,5-triphosphate (IP3). DAG remains bound to the membrane, and IP3 is released as a soluble structure into the cytosol. IP3 then diffuses through the cytosol to bind to IP3 receptors, particular calcium channels in the endoplasmic reticulum (ER). These channels are specific to calcium and only allow the passage of calcium to move through. This causes the cytosolic concentration of Calcium to increase, causing a cascade of intracellular changes and activity.[43] In addition, calcium and DAG together works to activate PKC, which goes on to phosphorylate other molecules, leading to altered cellular activity. End effects include taste, manic depression, tumor promotion, etc.[43]
History

The earliest scientific paper recorded in the MEDLINE database as containing the specific term signal transduction within its text was published in 1972.[44]
Some articles published before 1977 use the term signal transmission or sensory transduction in their titles or abstracts[45][46] but it was not until 1977 that papers began to be published with the specific term signal transduction in their abstracts, and it was not until 1979 that the term appeared within a paper title.[47][48]. One source attributes the widespread use of the term signal transduction to a 1980 review article by Rodbell.[49][50] As can be seen from the graph to the right, research papers directly addressing signal transduction processes began to appear in large numbers in the scientific literature in the late 1980s and early 1990s.
One notable early discovery in the field of signal transduction was the link Rodbell made between metabolic regulation and the activity of GTP and GTP-binding proteins.[50] The current understanding of signal transduction processes reflects contributions made over many years by research groups all over the world.
A total of 48,377 scientific papers related to signal transduction were published in 1977; of these, 11,211 were reviews of other papers.
See also
- Two-component regulatory system in microbes
- Functional selectivity
- G protein-coupled receptor -- GTPases -- Protein phosphatase
- Redox signaling
- transduction (distinctive sense: viral transduction)
References
- ↑ 1.0 1.1 Reece, Jane; Campbell, Neil (2002). Biology. San Francisco: Benjamin Cummings. ISBN 0-8053-6624-5.
- ↑ Beato M, Chavez S and Truss M (1996). "Transcriptional regulation by steroid hormones". Steroids 61 (4): 240–251. doi:10.1016/0039-128X(96)00030-X. PMID 8733009.
- ↑ Hammes SR (2003). "The further redefining of steroid-mediated signaling". Proc Natl Acad Sci USA 100 (5): 21680–2170. doi:10.1073/pnas.0530224100. PMID 12606724.
- ↑ Sugden D, Davidson K. et al. (2004). "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Res. 17 (5): 454–460. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
- ↑ 5.0 5.1 Carpenter G, and Cohen S. (1990). "Epidermal growth factor". J. Biol. Chem. 265 (14): 7709–7712. PMID 2186024.
- ↑ 6.0 6.1 Ward M, and Marcey, D. [1] Retrieved on 2007-03-06
- ↑ Schroder et al. (2004). "Interferon-γ an overview of signals, mechanisms and functions". Journal of Leukocyte Biology 75: 163–189. doi:10.1189/jlb.0603252. PMID 14525967. http://www.jleukbio.org/cgi/content/full/75/2/163.
- ↑ Chung CW, Cooke RM et al. (1995). "The three-dimensional solution structure of RANTES". Biochemistry 34 (29): 9307–9314. doi:10.1021/bi00029a005. PMID 7542919.
- ↑ 9.0 9.1 Kistler J, Stroud RM et al. (1982). "Structure and function of an acetylcholine receptor". Biophys. J. 37 (1): 371–383. doi:10.1016/S0006-3495(82)84685-7. PMID 7055628.
- ↑ Wiesmann, C. and de Vos, AM. (2001). "Nerve growth factor: structure and function". Cell Mol Life Sci 58 (5-6): 748–759. doi:10.1007/PL00000898. PMID 11437236.
- ↑ Goldstein, A. (1976). "Opioid peptides endorphins in pituitary and brain". Science 193 (4258): 1081–1086. doi:10.1126/science.959823. PMID 959823.
- ↑ Kroeze WK, Kristiansen K, and Roth BL. (2002). "Molecular biology of serotonin receptors, structure and function at the molecular level". Curr Top Med Chem 2 (6): 507–528. doi:10.2174/1568026023393796. PMID 12052191.
- ↑ Missale C, Nash SR. et al (1998). "Dopamine receptors:from structure to function". Physiol. Rev. 78 (1): 189–225. PMID 9457173.
- ↑ Adams TE, Epa, VC et al (2000). "Structure and function of the type 1 insulin-like growth factor receptor". Cell Mol Life Sci 57 (7): 1050–1093. doi:10.1007/PL00000744. PMID 10961344.
- ↑ Roy AK and Chatterjee B. (1995). "Androgen action". Crit Rev Eukaryot Gene Expr. 5 (2): 157–176. PMID 8845582.
- ↑ Small KM, McGraw DW and Liggett SB. (2003). "Pharmacology and physiology of human adrenergic receptor polymorphisms". Annu Rev Pharmacol Toxicol 43: 381–411. doi:10.1146/annurev.pharmtox.43.100901.135823. PMID 12540746.
- ↑ Burns ME and Arshavsky VY. (2005). "Beyond counting photons: trials and trends in vertebrate visual transduction". Neuron 48 (3): 387–401. doi:10.1016/j.neuron.2005.10.014. PMID 16269358.
- ↑ Ronnett GV and Moon C. (2002). "G proteins and olfactory signal transduction". Annu Rev Physiol 64: 189–222. doi:10.1146/annurev.physiol.64.082701.102219. PMID 11826268.
- ↑ Wong GT, Gannon KS and Margolskee RF. (1996). "Transduction of bitter and sweet taste by gustducin". Nature 381 (6585): 796–800. doi:10.1038/381796a0. PMID 8657284.
- ↑ Hanna MH, Nowicki JJ and Fatone MA (1984). "Extracellular cyclic AMP (cAMP) during development of the cellular slime mold Polysphondylium violaceum: comparison of accumulation in the wild type and an aggregation-defective mutant". J Bacteriol 157 (2): 345–349. PMID 215252.
- ↑ Sprague GF Jr. (1991). "Signal transduction in yeast mating: receptors, transcription factors, and the kinase connection". Trends Genet 7 (11-12): 393–398. doi:10.1016/0168-9525(91)90218-F. PMID 1668192.
- ↑ Lalli E and Sassone-Corsi P (1994). "Signal transduction and gene regulation: the nuclear response to cAMP". J Biol Chem 269 (26): 17359–17362. PMID 8021233.
- ↑ 23.0 23.1 Rosen O (1987). "After insulin binds". Science 237 (4821): 1452–1458. doi:10.1126/science.2442814. PMID 2442814.
- ↑ Guo D, Jia Q. et al (1995). "Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation". J Biol Chem 270 (12): 6729–6733. doi:10.1074/jbc.270.12.6729. PMID 7896817.
- ↑ Bornfeldt KE, Raines EW. et al. (1995). "Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation.". Ann N Y Acad Sci 766: 416–430. doi:10.1111/j.1749-6632.1995.tb26691.x. PMID 7486687.
- ↑ Massague J and Gomis RR (2006). "The logic of TGFbeta signaling". FEBS Lett 580 (12): 2811–2820. doi:10.1016/j.febslet.2006.04.033. PMID 16678165.
- ↑ Johansson S. Svineng G et al. (1997). "Fibronectin-integrin interactions". Front. Biosci 2: d126–146. PMID 9159220.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer; Web content by Neil D. Clarke (2002). Biochemistry. San Francisco: W.H. Freeman. ISBN 0-7167-4954-8.
- ↑ Yang W, Xia S (2006). "Mechanisms of regulation and function of G-protein-coupled receptor kinases". World J Gastroenterol 12 (48): 7753–7. PMID 17203515.
- ↑ Burger M, Burger, JA et al (1999). "Point mutation causing constitutive signaling of CXCR2 leads to transforming activity similar to Kaposi's sarcoma herpesvirus-G protein-coupled receptor". J. Immunol. 163 (4): 2017–2022. PMID 10438939.
- ↑ 31.0 31.1 Li E, Hristova K (2006). "Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies". Biochemistry 45 (20): 6241–51. doi:10.1021/bi060609y. PMID 16700535.
- ↑ Schlessinger, J. (1988). "Signal transduction by allosteric receptor oligomerization.". Trends Biochem Sci 13 (11): 443–7. doi:10.1016/0968-0004(88)90219-8. PMID 3075366.
- ↑ Roskoski, R, Jr. (2004). "The ErbB/HER receptor protein-tyrosine kinases and cancer.". Biochem. Biophys. Res. Commun. 319 (1): 1–11. doi:10.1016/j.bbrc.2004.04.150. PMID 15158434.
- ↑ 34.0 34.1 Hehlgans, S. Haase, M. and Cordes, N. (2007). "Signaling via integrins: Implications for cell survival and anticancer strategies". Biochim. Biophys. Acta. 1775 (1): 163–180. doi:10.1016/j.bbcan.2006.09.001. PMID 17084981.
- ↑ Gilcrease MZ. (2006). "Integrin signaling in epithelial cells". Cancer Lett. 247 (1): 1–25. doi:10.1016/j.canlet.2006.03.031. PMID 16725254.
- ↑ Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003). "Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway". Science 301 (5633): 640–3. doi:10.1016/j.canlet.2006.03.031. PMID 12855817.
- ↑ Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S (2003). "TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway". Nat Immunol 4 (11): 1144–50. doi:10.1016/j.canlet.2006.03.031. PMID 14556004.
- ↑ Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S (2002). "Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4". Nature 420 (6913): 324–9. doi:10.1016/j.canlet.2006.03.031. PMID 12447441.
- ↑ Delbridge L, O'Riordan M (2007). "Innate recognition of intracellular bacteria". Curr Opin Immunol 19 (1): 10–6. doi:10.1016/j.coi.2006.11.005. PMID 17126540.
- ↑ Fujita T, Onoguchi K, Onomoto K, Hirai R, Yoneyama M. "Triggering antiviral response by RIG-I-related RNA helicases". Biochimie. doi:10.1016/j.canlet.2006.03.031. PMID 17379377.
- ↑ David S. Goodsell (October 2004). "G Proteins: Relaying the Signal". Molecule of the Month. RCSB Protein Data Bank. http://www.pdb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/pdb58_2.html. Retrieved 2008-05-25.
- ↑ Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W (Dec 2005). "Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway". The Biochemical journal 392 (Pt 2): 249–61. doi:10.1042/BJ20050908. PMID 16293107.
- ↑ 43.0 43.1 Alberts B, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1.
- ↑ Rensing, L. (1972). "Periodic geophysical and biological signals as Zeitgeber and exogenous inducers in animal organisms". Int. J. Biometeorol. 16: Suppl:113–125. PMID 4621276.
- ↑ Tonndorf J. (1975). "Davis-1961 revisited. Signal transmission in the cochlear hair cell-nerve junction". Arch. Otolaryngol. 101 (9): 528–535. PMID 169771.
- ↑ Ashcroft SJ, Crossley JR, Crossley PC. (1976). "The effect of N-acylglucosamines on the biosynthesis and secretion of insulin in the rat". Biochem. J. 154 (3): 701–707. PMID 782447.
- ↑ Hildebrand E. (1977). "What does Halobacterium tell us about photoreception?". Biophys. Struct. Mech. 3 (1): 69–77. doi:10.1007/BF00536457. PMID 857951.
- ↑ Kenny JJ, Martinez-Maza O. et al (1979). "Lipid synthesis: an indicator of antigen-induced signal transduction in antigen-binding cells". J. Immunol. 112 (4): 1278–1284. PMID 376714.
- ↑ Gomperts, BD.; Kramer, IM. Tatham, PER. (2002). Signal transduction. Academic Press. ISBN 0-12-289631-9.
- ↑ 50.0 50.1 Rodbell, M. (1980). "The role of hormone receptors and GTP-regulatory proteins in membrane transduction". Nature 284 (5751): 17–22. doi:10.1038/284017a0. PMID 6101906.
Further reading
- Non-technical
- Werner R. Loewenstein, The Touchstone of Life: Molecular Information, Cell Communication, and the Foundations of Life, Oxford University Press, 1999, ISBN 0-19-514057-5
- Technical
- Gomperts, Kramer, Tatham, "Signal Transduction", AP/Elsevier 2nd edition [2009], ISBN 0-12-369441-8. Reference book, for more information: http://www.CellBiol.net.
- Gerhard Krauss, Biochemistry of Signal Transduction and Regulation, Wiley-VCH, 1999, ISBN 3-527-30378-2
- John T. Hancock, Cell Signalling, Addison-Wesley, 1998 ISBN 0-582-31267-1
External links
- Netpath - A curated resource of signal transduction pathways in humans
- Signal Transduction - The Virtual Library of Biochemistry and Cell Biology
- TRANSPATH(R) - A database about signal transduction pathways
- Science's STKE - Signal Transduction Knowledge Environment, from the journal Science, published by AAAS.
- MeSH Signal+Transduction
- UCSD-Nature Signaling Gateway, from Nature Publishing Group
- LitInspector - Signal transduction pathway mining in PubMed abstracts
- Huaxian Chen, et al. A Cell Based Immunocytochemical Assay For Monitoring Kinase Signaling Pathways And Drug Efficacy (PDF) Analytical Biochemistry 338 (2005) 136-142
- www.Redoxsignaling.com
|
|||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||